Multi-site clinical trial management for clinical research and clinical care
Clinical trials and clinical care for personalized therapies and complex disease areas, such as serious cancer, are today often managed by several centers and the interaction of different clinical focuses. Smooth, centralized exchange and seamless collaboration between clinical care and clinical research in real time and across multiple sites is essential for this.
Healex ClinicalSite provides secure, real-time collaboration for researchers and physicians
Healex ClinicalSite is the leading communication platform for information and data exchange between investigators, researchers and sponsors in clinical trials. Over 6000 researchers and 350 hospitals use the platform to manage clinical data with the highest data protection standards.
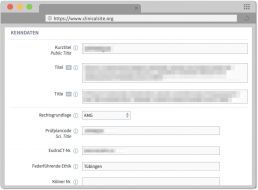
With Healex ClinicalSite, researchers and physicians can manage clinical studies across all phases and integrate and control involved parties with assigned roles. For the presentation and maintenance of study content, all legal and regulatory aspects of study management can be entered and managed, e.g. protocol codes, trial sites, trial objectives, inclusion criteria and patient characteristics, study design, recruitment duration and prescribed responsible roles such as investigators, clinical trial managers, sponsors and monitoring.
Leading communication platform
for information and data exchange between physicians and researchers in clinical studies
Multi-site information and data management system
for clinical study sites, coordinating centers and research networks
Central researcher and physician directory
for all study groups and researchers involved in a study with access management through roles and rights management
Core functions
Efficient trial management
- Synopsis
- Indications and substances
- Protocols and important documents
- Recruitment status of the study
- Timepoints of the study
- Collection of study organisation data: e.g. therapy scheme, patient collective, study characteristics, responsibilities, status and schedule of the study

Multi-site collaboration
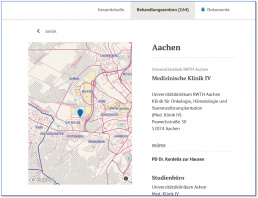
- Definition of responsibilities (sponsor / study group, study site)
- Time frame and definition of individual roles at study and project level
- Collection of contact and address data
- Administration of the members of an organisational unit
- Illustration of relationships to other organisational units
- Administration of network partners
- Release of the organisation members' documents and reports
- Integration of secondary systems
Easy publishing
- Publication and administration of local and multi-site studies
- Extensive options for filtering and exporting data
- Option to embed SMS/ClinicalSite into your own websites
- Options for access and export via API
- Local and central document management
- Administration of network partners
- Option to export the study groups
- Option to upload study documents
- Publication of study data via API
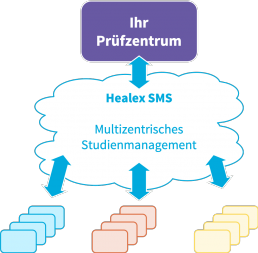
Seamless integration
- Software as a Service (SaaS): Flexible access from internet enabled systems and mobile devices
- Secure access management through OpenID Connect interface
- Retrieval of study administration data via secure API
Low system requirements
- PC / Mac / Tablet with internet connection
- Cookies must be accepted
- usually corresponds to the default settings of the browser
- the system does not use the cookies for any other purpose
- Activation of Javascript
- usually corresponds to the default settings of the browser
- Standard-compliant browser
- Firefox
- Apple Safari
- Opera
- Google Chrome
- Microsoft Edge
Support
- Support hours: 08am – 6pm
- Hotline: +49 (0)2203 5026369
- E-Mail: support@clinicalsite.org
- Dokumentation: ClinicalSite Manual (in German)